
Carotenoid extracted from A, B, C and D using MgSilicate, CaSilicate,... Download Scientific
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Chloroform SRF / Gujrat at Rs 65/kg Chloroform in Delhi ID 23920489648
Chloroform, or trichloromethane (often abbreviated as TCM ), is an organic compound with the formula C H Cl 3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and in turn PTFE. [10]
PPT Polar Bonds and Molecules PowerPoint Presentation ID3762676
CHCl3 is an organic compound known by its IUPAC name as trichloromethane. It is also commonly known by the term chloroform. It exists as a colorless dense liquid having a sweet smell. Many of you might have doubts regarding whether CHCl3 is polar or not.

Liquid Chloroform at Rs 55/kg Pune ID 2851062413262
Answer Verified 262.2k + views Hint: Chemical elements are the purest form of atoms. Atoms combine to form molecules. Chloroform is a molecule with the atoms of carbon, chlorine and hydrogen.

Chloroform's Gallery Pixilart
Expand/collapse global location. 6.05.1. Protic vs Aprotic Solvents. Page ID. Solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic. Because non-polar solvents tend to be aprotic,the focus is upon.

Chf3 Polar Or Nonpolar Asking List
Chloroform | CHCl3 | CID 6212 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.. It has a role as an inhalation anaesthetic, a non-polar solvent, a carcinogenic agent, a central nervous system drug and a.

Chloroform Lab preparation, Properties, Uses and Question/Answer
About Transcript Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.

Chloroform Polarity of Chloroform
Thin Layer Chromatography (TLC) is an extremely useful technique for monitoring reactions. It is also used to determine the proper solvent system for performing separations using column chromatography. TLC uses a stationary phase, usually alumina or silica, that is highly polar (standard) or non-polar (reverse phase), and a mobile phase, some.

Best Overview Is CHCl3 Polar or Nonpolar Science Education and Tutorials
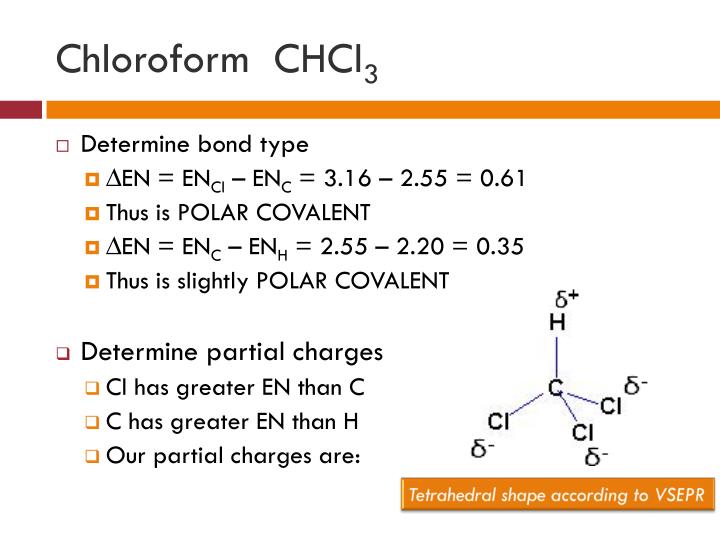
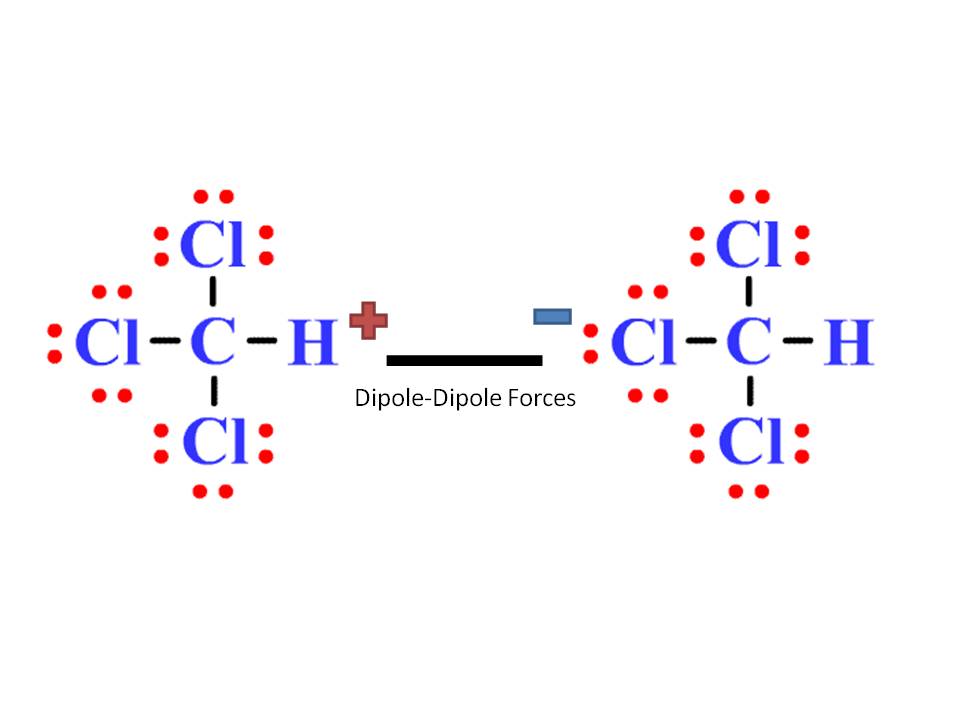
Chloroform (CHCl3) is a polar molecule. Chlorine (Cl) is a highly electronegative element. It attracts the shared electron cloud of each of the three C-Cl bonds as well as the C-H bond. Oppositely charged poles develop in the molecule. CHCl3 has an apparently symmetric tetrahedral shape or geometry.

Chloroform, 1 liter Lösningsmedel Tillbehör HPLC Kromatografitillbehör Produkter
Lipids are all insoluble in polar solvents like water but highly soluble in the non-polar or weakly polar organic solvents, including ether, chloroform, benzene, and acetone. In fact, these four solvents are often referred to as "lipid-solvents" or "fat-solvents". Other biomolecules such as amino acids, proteins, carbohydrates, and nucleic.
The theoretical values of the IPCE and ΔGinj (a) in polar solvents,... Download Scientific Diagram
Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it." Nonetheless, guidelines have been created to make it easier.

Nonpolar and polar lipid structures which represent lipids found... Download Scientific Diagram
The polarity of a Molecule. The molecules fall into the following categories concerning molecular polarity. The molecule is nonpolar if there is no polar bond in it, e.g., H-H, F-F, and CH 4 are nonpolar molecules. Fig. 3.8.4 illustrates CH 4 molecules with green color electron clouds that represent a nonpolar molecule.. Figure \(\PageIndex{4}\): Methane (CH 4) with no polar bond is nonpolar.
Chloroform
For example, carbon tetrachloride, CCl 4, is nonpolar, but chloroform, CHCl 3, and methyl chloride, CH 3 Cl are polar:. The table below shows whether the examples in the previous sections are polar or nonpolar. For species which have an overall charge, the term "charged" is used instead, since the terms "polar" and "nonpolar" do.
The Chemistry of Chloroform
Chloroform the molecule is polar, as you said. Chloroform the solvent is "nonpolar" because it has a low dielectric constant. Check in wiki here - you can see that chloroform has a dipole moment that is not super high, but nonzero, however its dielectric constant is much lower than those solvents classified as "polar". 3.

Chloroform, Packaging Type HDPE Barrel, 220 kg at Rs 45/kg in Coimbatore
Polarity of Solvents. Water Acetic Acid Ethyleneglycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diehylamine Aniline Dimethylsulfoxide Ethylacetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane.

Chloroform Stock Footage & Videos 13 Stock Videos
5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions.